Call to Schedule Free Consultation at Over 45 Centers Worldwide!
+1844 GET-STEM
+1844 GET-STEM
Receive first rate stem cell and exosome therapy from the world’s leading regenerative medicine providers. Over 25,000 procedures in 7 countries in the past decade with 85% patient satisfaction. Book your consultation to see if you or a loved one is a candidate! You could be the next stem cell success story!
Register Now For Free Webinar: Avoid Surgery With Stem Cell Therapy
Wouldn’t it be great if your body’s repair mechanisms (stem cells, growth factors, cytokines) can be harnessed to repair tissue injuries without surgery, giving you the ability to do things that you want to do, like play with your kids again, go hiking, swimming, ride a bike, and be… pain free!
R3’s Centers of Excellence offer over 20 customized protocols for patients with stem cells, exosomes, PRP, vitamins, peptides and more to help you achieve a BETTER quality of life.
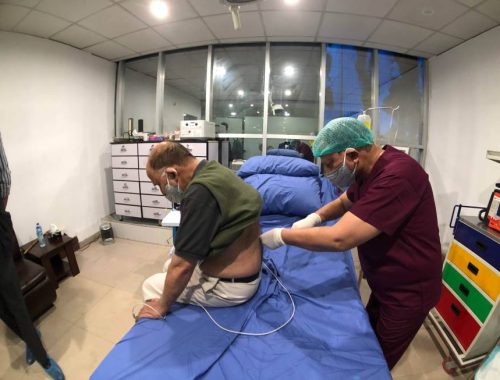
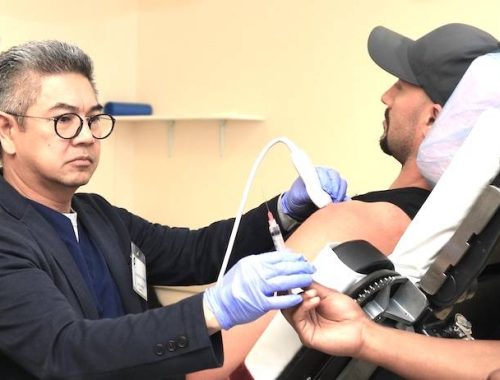

We answer your most frequently asked questions about regenerative medicine therapies.










Download our FREE consumer guide. FAQ’s are answered and will help you make more informed decisions about your regenerative options!
Guide
Consumer guide for stem cell and exosome therapy
I Used To Hear It All The Time. “Stem Cells Are A Fad. Stem Cells Don’t Work. There’s Not Enough Evidence Yet. You Shouldn’t Offer Something That’s Not Fda Approved.”

Our Centers change lives everyday. Patients frequently achieve relief from pain while getting back to desired activities such as walking farther, golfing, playing with the kids or grandkids, and participating in recreational activities.*
The most revolutionary regenerative medicine therapies now being offered include umbilical cord tissue and exosomes. These biologics contain growth factors, hyaluronic acid, cytokines, exosomes and stem cells that are potent, active, and viable.
R3 Stem Cell’s Centers of Excellence have performed over 25,000 regenerative procedures globally. The umbilical tissue and exosomes are obtained ethically and processed at cGMP and ISO Certified labs that have excellent safety records.

*No patients were paid for their testimonials.
With a third of the US suffering from chronic pain, and over a million each year undergoing joint replacements in the USA, regenerative therapies have a large target audience. R3 Stem Cell has achieved Institutional Review Board (IRB) Approval for the investigation of regenerative therapies including orthopedic and autoimmune conditions. See the specifics on ClinicalTrials.gov HERE.
See Arnel’s story by clicking on the video, he performed just hours after his procedure!
Success Stories
*Outcomes will vary between individuals. No claims are being made with regenerative therapies. The FDA considers stem cell therapy experimental. See our THERAPY COMMITMENT HERE.
The USA Stem Cell Leader Offers Procedures In 7 Countries Including

United States

Mexico

Philippines

South Africa

Pakistan

India

Turkey
Call to schedule your free consultation at over 70 centers world!
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiu tempor incididunt ut labore et dolore magna aliqua. Ut enim ad mi veniam
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiu tempor incididunt ut labore et dolore magna aliqua. Ut enim ad mi veniam
R3 Stem Cell offers financing options for North American residents, wherever they obtain procedures!
R3 Stem Cell is a global leader in regenerative medicine, offering advanced stem cell and exosome therapies through Centers of Excellence in over 45 locations across 7 countries. Our mission is to provide first-rate, customized regenerative treatments, including stem cells, exosomes, PRP, vitamins, and peptides, to help patients achieve a better quality of life. With more than 26,000 procedures performed and an 85% patient satisfaction rate, our experienced, compassionate providers are committed to delivering safe, effective care. All therapies are nonsteroidal, outpatient procedures designed to harness the body’s natural repair mechanisms and support tissue regeneration. Individual results may vary, and only your medical professional can explain all the risks and potential benefits of any therapy based on your unique circumstances.
Stem cell therapy is considered experimental and is regulated by the U.S. Food and Drug Administration (FDA), but it is not FDA-approved. R3 Stem Cell does not offer stem cell therapy as a cure for any medical condition. No statements made on this site have been evaluated or approved by the FDA. This site does not provide medical advice. All content is for informational purposes only and is not a substitute for professional medical consultation, diagnosis, or treatment. Reliance on any information provided by R3 Stem Cell, its employees, others appearing on this website at the invitation of R3 Stem Cell, or other visitors to the website is solely at your own risk. R3 Stem Cell does not recommend or endorse any specific tests, products, procedures, opinions, or other information that may be mentioned on this website. R3 Stem Cell is not responsible for the outcome of your procedure. The FDA considers stem cell therapy experimental at this point.
R3 has treatment centers in 8 countries. In the USA, no claims are made for treating any specific condition. Those are meant for R3 International treatment.










CALIFORNIA
FLORIDA
GEORGIA
HAWAII
IDAHO
ILLINOIS
INDIANA
IOWA
KANSAS
KENTUCKY
LOUISIANA
MARYLAND
MASSACHUSETTS
MICHIGAN
MINNESOTA
MISSISSIPPI
MISSOURI
NEBRASKA
NEW JERSEY
NEW YORK
NEW MEXICO
NEVADA
NORTH CAROLINA
OHIO
OKLAHOMA
OREGON
PENNSYLVANIA
RHODE ISLAND
SOUTH CAROLINA
SOUTH DAKOTA
TENNESSEE
Copyright © 2017-2025 R3 Stem Cell. All Rights Reserved.